Pharmaceutical industry
Project aims
This ongoing project seeks to explore possible future global value network configurations for the pharmaceutical industry that align with a disruptive switch in technology from batch-based manufacturing processes to continuous process manufacturing. It is a phased multi-year project involving a cross-sector consortium tailoring and applying the CIM approach. One key aspect is the integration of critical sub-systems within the total network.
Approach and key findings
A four-step process was used to identify alternative value network opportunities.
POTENTIAL OPPORTUNITIES
- Plant footprint reduction by 70%
- CapEx reduction by 25%
- Operating cost reduction by 30%
- Yield improvement by 10%
- More consistent quality
- More controllable, repeatable processes
POTENTIAL BARRIERS TO ADOPTION
- Regulatory uncertainties
- Under-utilisation of existing capacity
- Technological readiness and uncertainties
- No clear and specific vision as to how continuous manufacturing may impact industry structure
- Transformation challenge and behavioural issues
Step 1: Identifying potential opportunities, barriers and target markets
Initial research identified a number of opportunities for the implementation of continuous manufacturing in the pharmaceutical industry, and potential barriers to their adoption (as illustrated in the table below). The aim of the project is a highly ambitious step-change in operational performance. The barriers, however, are significant, and require a coordinated and systematic approach to redesigning the entire value network.
Potential benefits of continuous manufacturing in the pharmaceutical industry
The analysis identified, at a conceptual level, the potential end-user target markets and products where continuous processing technology might provide attractive value network opportunities, either in meeting unmet user needs or providing step changes in cost, flexibility or reliability.
Step 2: current state mapping and definition of critical sub-systems
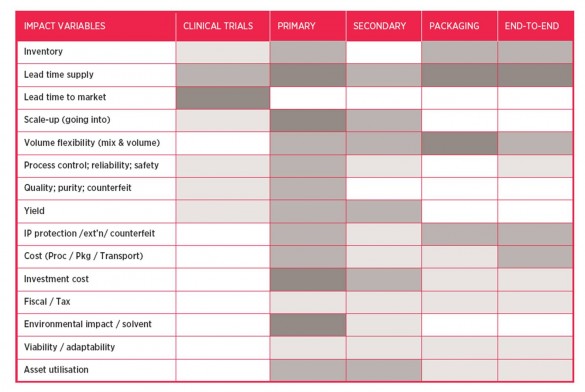
The second step involved mapping the current state of the batch-based global value network for the pharmaceutical industry (using the mapping approach described in section 1.2). This led to the identification of the critical sub-systems that would be affected by the shift to continuous manufacturing. Initial analysis was conducted to identify the potential of the shift for each sub-system.
Step 3: sub-systems analysis against desired benefits
Deeper analysis of the sub-systems was then carried out to support a tailored future configuration aligned with the specific benefits identified in step 2. The graphic below illustrates the outputs, where the five graphics refer to the five sub-systems. The ‘bubbles’ in each graph represent the different product-process segments making up each sub-system. Shaded areas signify attractive boundary conditions for continuous processing. This analysis supported deeper understanding of the ideal future configuration for each sub-system.
Step 4: integration of the critical sub-systems
The final step of the process guided the integration of the sub-systems. This involved detailed examination of the interactions between the five areas (clinical, primary/secondary manufacturing, packaging and distribution, end-to-end supply) to identify target applications for continuous manufacturing that could work within and across the sub-systems. The target applications were then assessed in terms of different transformation scenarios, bringing together inputs on technology readiness and business viability.
Future work
This study is part of an ongoing research agenda that seeks to understand future disruptive changes in the pharmaceutical industry global value network. This involves extending the preliminary analysis described above to cover other key areas in terms of patient populations and product-process segments. This work will seek to explore the attractive business propositions alongside technological feasibility, and will consider the behavioural changes and dynamic capabilities required to make the transformation across the total value network.
Capturing value from global networks
Strategic approaches to configuring international production, supply and service operations
ISBN: 978-1-902546-30-8









