Diagnosing deadly diseases

University of Cambridge engineers and scientists from Fluids in Advanced Manufacturing and Cambridge Analytical Biotechnology groups have joined forces to develop an on-the-spot low-cost tool for the rapid diagnosis of infectious diseases. Research team member and Gates Cambridge Scholar, Cassi Henderson, explains how the new technology could save lives in developing countries where there is little or no access to medical pathology laboratories and specialist technicians.
Treatable infectious diseases are among the major causes of death in low- and middle-income countries. One of the main problems is that some infectious diseases share similar or identical symptoms and often the only way to confirm the illness is to take a blood sample and run a series of specialist diagnostic tests in a laboratory.
The potentially life-threatening bacterial disease leptospirosis is one such infection that produces febrile symptoms that are difficult to distinguish from other illnesses such as dengue fever, typhoid fever, malaria and viral hepatitis. Leptospirosis can be cured, but an individual’s chances are much improved if the disease is diagnosed and then treated with antibiotics within the first five to seven days of infection.
Cassi Henderson in the lab
Accurate diagnosis requires a blood test to be analysed in a pathology laboratory. As a result, leptospirosis is often under-diagnosed and has a high mortality rate in low-income countries. It has been recognised as a neglected disease by the World Health Organization (WHO), which has established a reference group to direct global research and action to address it.
It is critical that health practitioners are able to detect the difference between bacterial and viral infections like leptospirosis and dengue fever because they require very different treatments. This is especially important in light of the rise in global antibiotic resistance with clinicians reluctant to administer antibiotics without a confirmed diagnosis.
Combining biotechnology and manufacturing
To address this, we are using innovative biotechnology and manufacturing technologies to develop a low-cost test that can deliver a real-time result without the need to send blood samples off to a centralised pathology laboratory or the need for specialist technicians to process the samples, both of which are in short supply in low- and middle-income countries.
The aim is to integrate the whole testing process into a single instrument similar to the home pregnancy test, known as a point-of-care diagnostic test. Rather than needing a medical practitioner to take a full blood sample from the patient, the instrument will only require a small finger-prick blood sample. The first diagnostic test that we are developing will detect leptospirosis, with the plan to adapt the system to detect other infectious diseases in the future.
Diagnosis methods
There are two primary ways to test for pathogens in blood: testing for the body’s delayed immune response to the presence of a pathogen (immunoassays) or directly testing for the presence of the pathogen’s genetic material (nucleic acid tests that detect DNA for bacterial infections or RNA in the case of viruses). Directly testing for the DNA/RNA of the pathogen can enable earlier detection than immunoassays.
However, the total amount of pathogenic DNA/RNA in a small finger-prick of blood can be quite low, particularly in the early stages of infection. To find a pathogen in such a small blood sample we need a way to replicate the pathogen’s DNA/RNA (amplification) to a high enough level that it can be detected by a diagnostic test.
The standard laboratory process used for nucleic acid amplification is a ‘polymerase chain reaction’ (PCR), but PCR testing usually requires specialist laboratory based equipment.
Alternative DNA replication
We are working to customise an alternative nucleic acid amplification method that has higher amplification efficiency and requires simpler equipment to perform the test. Our current focus is on designing the enzyme (the nucleic acid polymerase) that performs the amplification and its integration into a hand-held sample-to-answer diagnostic test.
Local and low-cost manufacturing
Whatever test we design must also be affordable if it is to be used in low-income countries. A barrier to low-cost diagnostics in the developing world arises from long supply chains and logistical challenges where medical equipment has to be transported long distances and to remote locations. The other challenge that is key here is the purchasing power parity.
To overcome this, we are using novel technologies to enable the diagnostic tests to be manufactured locally, targeting less than $0.50 per test.
One of the more expensive components of the test is the enzyme that is used to amplify the pathogen’s genetic material (DNA/RNA) to high enough levels to be detected from a finger-prick blood sample. The high cost and required specialist skills of traditional methods of manufacturing the enzyme, together with requirements for refrigeration of the product, would present a barrier to local production. To address this, we have developed a technique that allows for direct purification of the enzyme from cell culture that packages the enzyme ready for use and bypasses the complex multistep process.
The enzyme has been genetically modified using synthetic biology techniques so that it can be collected easily by sedimentation. The protein has also been given a pink colour to make it easy to see when the enzyme has been successfully produced and collected. These two features should make it possible for individuals with minimal training to manufacture the enzyme locally in low resource regions.
While critical to performance, the enzyme is just one component of a successful point-of-care test. In addition to manufacturing the enzyme, we are developing a novel technique to extract the pathogen’s DNA/RNA directly from the blood sample. This DNA/RNA extraction system and the enzyme will both be contained on a diagnostic card that can also be manufactured locally and ultimately manufactured with local materials. The diagnostic test will be linked to a mobile phone app for data collection and transmission when required.
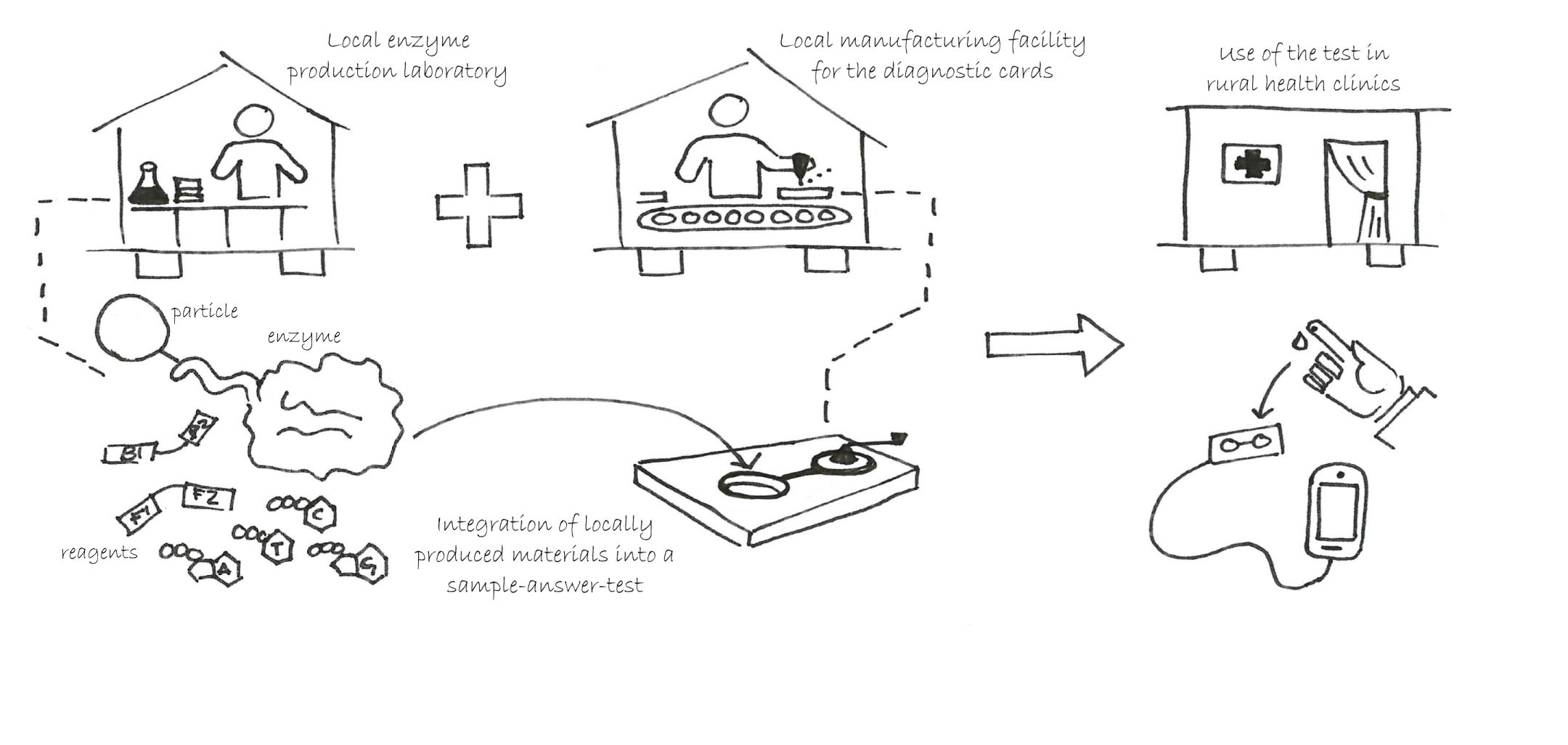
The diagnostic card containing the novel engineered enzyme and DNA/RNA extraction component is being designed so that it can be locally manufactured in low- and middle-income countries. The integrated test would pair with a mobile phone for data collection and transmission during its use in the field. © Cassi Henderson
Project leaders Professor Lisa Hall, Department of Chemical Engineering and Biotechnology, University of Cambridge (left) and Dr Ronan Daly, Head of the IfM’s Fluids in Advanced Manufacturing Group (right) with Gates Cambridge Scholar, Cassi Henderson (centre).
This project has brought together people from multi-disciplinary areas of expertise in diagnostics, synthetic biology, microbiology and parasitology, colloidal science and manufacturing engineering. We are also working in collaboration with Universiti Putra Malaysia and Professor Gordon Awandare, from the West African Centre for Cell Biology of Infectious Pathogens at the University of Ghana, to develop specific aspects of the system, but would welcome new partners in both the UK and internationally who would like to adapt these approaches to other diagnostic needs.

Professor Gordon Awandare (wearing a light blue lab coat) and his research team at the West African Centre for Cell Biology of Infectious Pathogens, University of Ghana, are working with the University of Cambridge to develop a low-cost tool for the rapid diagnosis of infectious diseases.
We hope to deliver a sustained improvement in healthcare, while also developing local economies, by using advances in synthetic biology and the application of latest manufacturing research to deliver a robust local fabrication set-up. This will also drive local enterprise, improve technological education and management of infection. We see this as a sustainable approach to point-of-care low-cost diagnostics.
Date published
12 May 2017










