Development and Optimisation of an Optofluidic Nano Tweezers System for Trapping Nanometre Crystals for Synchrotron X-Ray Diffraction Experiments
Introduction
With the Synchrotron community facing an increasing need to analyse ever-smaller protein crystals (particularly at the Diamond Light Source VMXm, I24 and I04 beamlines); new sample loading techniques are needed to present nano-dimensional samples to the beam for X-ray diffraction experiments.
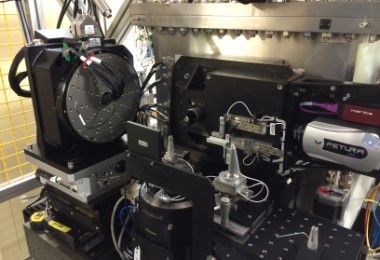
Figure 1. The I24 beamline hutch.
When dimensions are in nanometres, traditional approaches, such as mounting on micromeshes are neither feasible nor appropriate because the levels of solvent around the crystals becomes unacceptable from an X-ray signal-noise perspective, and visualization of the crystals by light microscopy becomes very challenging. Additionally, these techniques, if reconfigured to work with nano dimensional samples, only offer the potential to automate – leading to clumsy top down approaches such as robotic handling. Moderate success with visualizing these nanometre sized crystals has been achieved, by injecting a slurry of crystals past the detector with a hit rate in the region of 10% with the rest of the sample being wasted. This method also requires imaging to take place as the sample is in motion.
One approach being given serious consideration involves optical trapping. Pioneered by Arthur Ashkin, optical tweezers apply highly focused laser beams to trap and manipulate microscopic artefacts ranging from a few microns to tens of nanometres; allowing for individual cells, viruses and protein molecules to be successfully trapped.
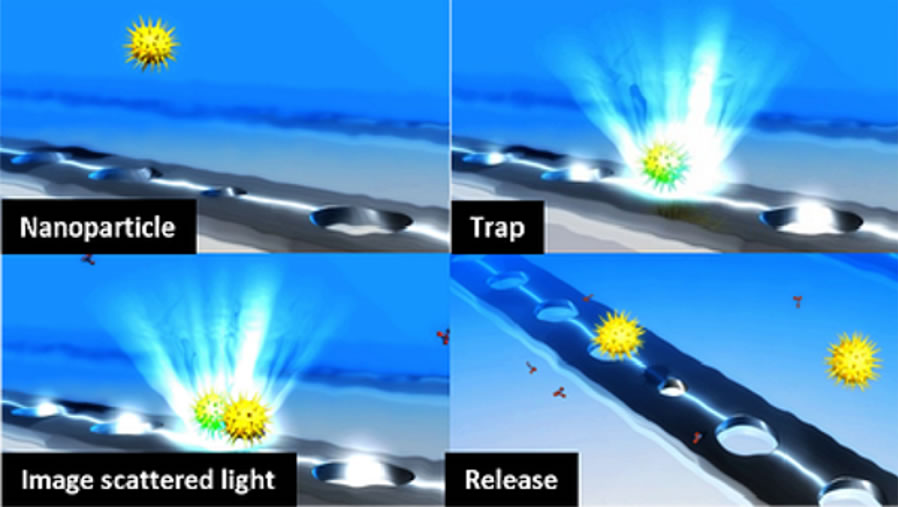
Figure 2. The Optofluidics particle trapping process. [1]
Specification
The system in this project generates a trapping field by harnessing the evanescent fields which arise from the total internal reflection of a 1064nm laser carrying waveguide. The individual specimens are transported by a microfluidics channel up to the waveguide where they are trapped and manipulated into the synchrotron’s beam path as it emerges from the beamline aperture. The optofluidic system which has been selected is commercially available; however, it is only designed for traditional light microscopy experiments, posing an exciting challenge.
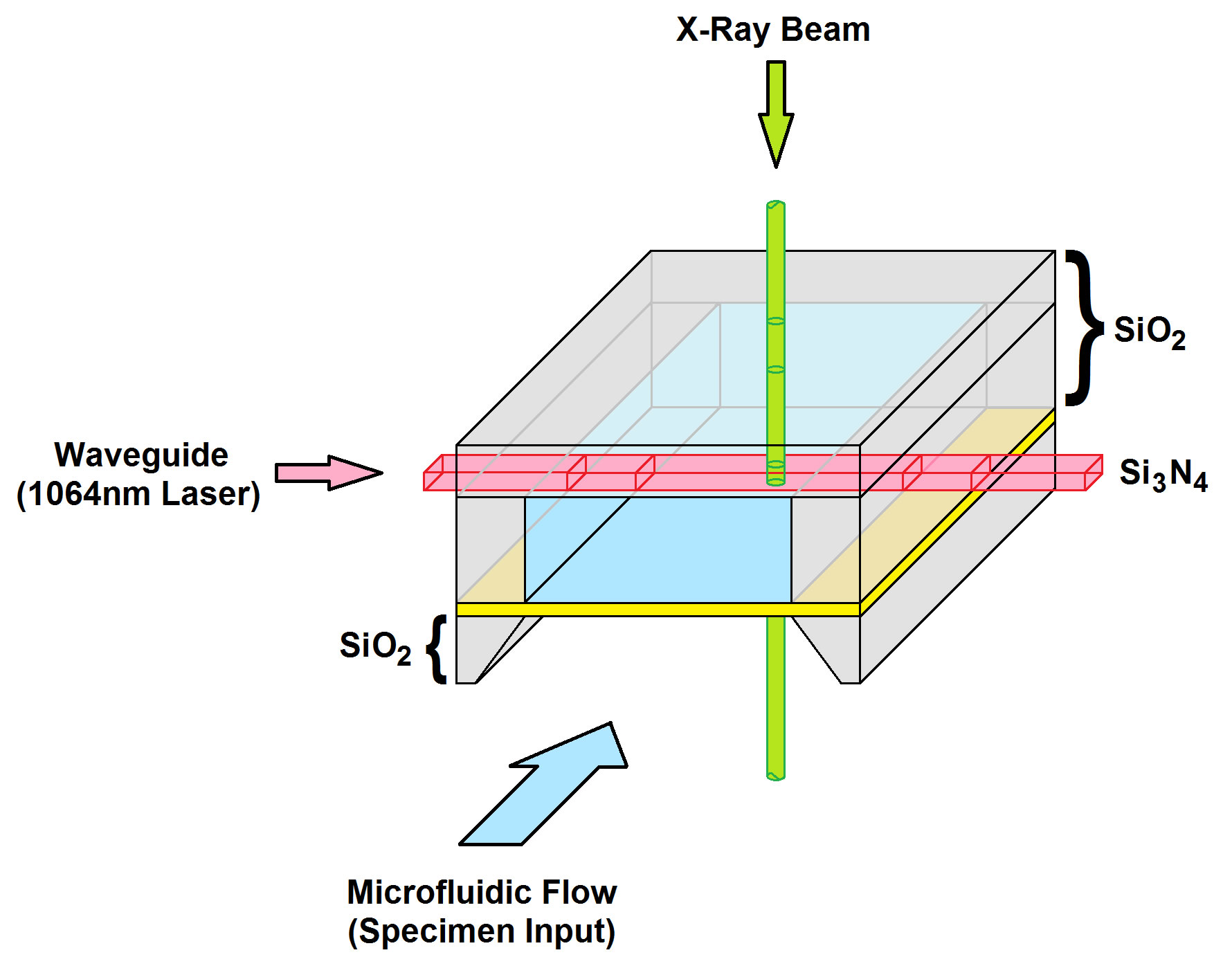
Figure 3. Diagram representing the optofluidic chip architecture.
Objectives
The results of this project aim is to implement a novel bottom-up approach, to substantially increase the precision of sample loading process, improve the speed and quality of measurement and to also reduce the amount of sample wastage – all goals which will lead to a reduction analysis of cost and time.

Figure 4. Photograph of 3 separate 400nm particles which have been successfully trapped above the waveguide. [2]
Researchers:
- Alex Diaz
- Prof. W. O’Neill
- Dr. M. Sparkes
- Dr. P. Docker, Diamond Light Source
Funders
Collaborators
References
[1] Optofluidics;Accessible on: http://www.opfluid.com/
[2] P. Docker, D. Axford, M. Prince, B. Cordovez, J. Kay, D. Stuart, G. Evans; “The Development of an Automated Nano Sampling Handling System for Nanometre Protein Crystallography Experiments”,
Diamond light Source, Harwell, OX11 0DE, UK; Research Gate; Jul 29, 2015; Accessible on:http://www.researchgate.net/publication/280551202_The_Development_of_an_Automated_Nano_Sampling_Handling_System_for_Nanometre_Protein_Crystallography_Experiments









