Collaboration as the best medicine
Collaboration can be pivotal in making change happen. So how can new collaborative models be forged in areas where such relationships are not well established, but where change is required?
In UK medicines manufacturing, there is an opportunity and a pressing need for progressive change. But there are also plenty of challenges and obstacles, particularly in a highly competitive and tightly regulated sector. A bold step forward has been taken over the past four years through a collaborative £23m project, ReMediES, involving 22 industry partners and two leading UK Universities.
As ReMediES concludes its work and passes on the baton, we talk with the IfM’s Dr Jag Srai, Research Director on the project, along with some of his colleagues in ReMediES, to reflect on how the project has forged a model for change through collaboration.
Medicines manufacturing is an important part of the UK economy, employing 40,000 people and generating $33 billion in exports. In many ways, it is productive, strong and successful. However, there is potential for making significant improvements across the medicines manufacturing supply chain.
The pharmaceutical industry’s size, scale and complexity, alongside its highly-competitive character, have made change difficult. There is an entrenched pattern of long product development cycles with high attrition rates, leading to development costs of around $2.6 billion per product. Clinical trials often take place many months after the trial drugs have been manufactured, resulting in significant stock write-offs of 50% or more. The commercial supply chain has become cumbersome and inventory-heavy. Furthermore, the sector is highly regulated, which instils caution.
While this status quo may sound problematic and somewhat bleak, in fact it has provided a much-needed stimulus for industry players to seek improvements, and a motivation to reach over those high competitive walls and collaborate for mutual benefit.
Remedies for change
Dr Jag Srai, Head of the Centre for International Manufacturing at the IfM, has been working with major players in the pharmaceutical industry for several years.
He comments:
“Medicines manufacturing needs to embrace new production and digital technologies that can support more responsive inventory-light supply chains, able to deliver medicines that are more targeted to specific patient groups or even personalised to individuals, with the potential to revolutionise healthcare delivery. This is too much for a single company, even a multinational, to achieve in isolation.
“So as a step in the right direction, we developed a vision for a large precompetitive collaborative project to drive change, with the active participation of the regulator.”
In 2014, the ReMediES (Reconfiguring Medicines End-to-End Supply) project was launched, bringing together 22 industrial partners including global pharmaceutical companies GlaxoSmithKline and AstraZeneca, as well as major contract manufacturing organisations, equipment manufacturers, international logistics specialists and a global pharmacy. The University of Cambridge and University of Strathclyde provided expertise in supply chain and pharmaceutical product and process engineering.
This broad partnership addressed some of the key challenges facing small molecule medicines manufacturing, with eight workstreams focusing on different aspects of clinical and commercial supply chains.
Jag, as Research Director of the project, explains:
“Across the ReMediES workstreams, we have attempted to tackle some ambitious questions. Can we run our clinical trial production more efficiently ‘on-demand’, reducing waste, inventory and lead-times? Can we adopt advanced technologies to make both our primary and secondary manufacturing processes right first time, more environmentally sustainable and capable of responding to changing demand? Can we apply science to create more soluble medicines? Can we package medicines in ways that will reduce wastage and help with patient compliance? And can we get better at delivering drugs to the people who need them, when they need them?”
Over its four-year timescale, the ReMediES partners worked to tackle these essential, cross-sector, cross-functional challenges. The partners were able to take fundamental and applied research through to prototype or commercialisation.
Dr Clive Badman, former Vice President of Pre-Competitive Collaboration at GlaxoSmithKline, was instrumental in forming the ReMediES consortium and became its Project Director. He comments:
“Many of the firms have experimented with some of the technologies we are talking about in ReMediES, but have not had the scale and the expertise that the ReMediES consortium offers to take things forward.”
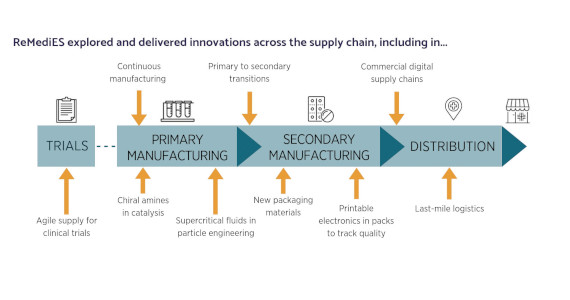
So what were the positive outputs from this collaborative approach? As the project reaches the end of its funding, partners have been reflecting and reporting on some of its key achievements.
Clinical supply chains – ‘just-in-time’
Clinical trials are a critical part of pharmaceutical R&D, but are increasingly complex to manage, often involving thousands of patients at hundreds of sites in different countries. At the same time, the sector wants to bring new medicines to market more quickly and more cheaply.
In the current system, it typically costs in excess of £75 million to run a clinical trial for a new drug; an enormous cost considering only 25% of drugs trialled make it into commercial production.
Because the sector is still reliant on high-volume production techniques it has to manufacture a trial drug in large quantities, often before the patients have been recruited, sometimes without confirmation that the trial is going ahead. Clinical teams have to decide doses and quantities of a trial drug to be manufactured 12 to 18 months before they expect to use it. As a result, more drugs are made than are needed to cover contingencies.
The process is also not sufficiently agile to respond to the changing circumstances inherent in any trial. For example, early results might suggest altering dosing or randomization strategies.
The ReMediES clinical trials workstream looked at how this could be improved, with the ambitious aim to cut supply time from 4-6 months to less than a week, and develop the ability to be agile to changing trial needs.
Project Lead, Andrew Dwyer from GSK, explains:
“We prototyped a new ‘just-in-time’ clinical pharmacy that can provide drugs to support complex drug trials, thereby reducing costs, increasing responsiveness and enabling a more flexible and exploratory approach to clinical research.
“The modelling of stock implications for a made-to-order facility has demonstrated that the potential benefit of an automated clinical pharmacy could be savings of tens of millions of pounds per year per company.”
Primary manufacturing – from batch to ‘continuous processing’
The challenges at the primary manufacturing stage are similar to those facing the clinical supply chain: a reliance on the overproduction of stock to mitigate a lack of agility in the supply chain, creating a high level of wastage. The sheer scale of batch manufacturing means that mistakes are expensive. If something goes wrong in a 10,000 litre vessel, the waste of energy and materials is significant. In continuous manufacturing, on the other hand, the process is constantly monitored and adjusted to ensure quality.
Through a number of related projects, ReMediES has made significant progress in developing new chemistries and processes that support the move from batch to continuous manufacturing, shrinking factory scale, increasing speed and reducing cost.
It has resulted in:
- New equipment for continuous processing with immediate commercial applications that support right-first-time manufacturing, yield improvements and inventory reduction.
- The development of a new GMP supercritical fluid manufacturing facility in the UK.
- Significant advances in biocatalysis and the use of enzymes in flow.
Secondary manufacturing
A major three-part project in secondary manufacturing has piloted new equipment for capabilities that enable shorter production cycles and volume flexibility.
Liz Meehan, responsible for Pharmaceutical Technology and Development at AstraZeneca, and workstream project leader for ReMediES, comments:
“A more flexible and agile medicines supply chain will be enabled by wider implementation of advanced (continuous or additive) manufacturing processes for both drug substance and drug product.”
The ReMediES project included user trials and case studies for some strategic technology platforms including continuous drug substance isolation, continuous direct compression, hot melt extrusion and 3D printing. As Liz explains:
“By taking a multidisciplinary approach, ReMediES has shown how these advanced manufacturing processes can be deployed and accelerated to meet the challenges of future clinical and commercial supply chains.”
The result will be substantial reductions in the cost of production, estimated in one company alone at £10m per year once fully implemented.
Smarter packaging
The ReMediES packaging workstream, in which AstraZeneca and GSK have collaborated closely, looked at using new materials and processing techniques to improve blister pack moisture barrier properties whilst at the same time reducing their size, leading to significant cost-savings and reductions in environmental impact.
This production process, now going to full trial, suggests a 10-fold improvement in moisture barrier compared with other similar cost materials. For one company alone it could mean, based on an example application for a leading product:
- A reduction in CO2 emissions of 700 tonnes annually.
- Savings of around £380k for materials and £1.5 million on distribution.
- A material productivity increase of 25%.
The ReMediES team, led by the Centre for Process Innovation (CPI) also developed smart labels for use on medicines packaging using printed electronics which enable product tracking, monitoring and could be used to support patient engagement. Successful demonstration is leading to commercialisation opportunities that are being pursued with leading packaging manufacturers.
Digitalisation of commercial supply chains
Several projects have underpinned the development of future supply chain models led by the team at the IfM.
Drawing data from 138 companies and 34 research organisations an asset library for use across the industry was developed in the form of a ‘capability matrix’ setting out what asset capabilities exist across the extended supply chain. This was then expanded using data-mining of commercial databases, to identify collaborative relationships in key capability areas. An interactive digital platform tool was created for use by ReMediES partners to interrogate the current collaborative network across the UK pharmaceutical landscape for specific asset capabilities, and to foster future collaboration.
A number of projects were conducted on the use of specific digital technologies across the end-to-end supply chain. These include an electronic patient-information leaflet, developed in consultation with the regulator. This is designed to co-exist or replace the paper version used in medicine packs with a prototype mobile phone app providing patients electronic access. The hope is that such innovations can increase patient compliance and reduce waste.
An end-to-end supply network design, analysis and modelling platform has also been developed to help manufacturers understand the opportunities available to reduce inventory and increase speed-to market. The multi-layer modelling platform allows organisations to evaluate new production processes, how these might influence production location and scale, inventory requirements of segmented product portfolios and market service levels.

The Supply Chain Reconfiguration Lab at the IfM
As Jag commented on the work in his Centre being led by Dr Ettore Settanni:
“We can slice and dice a range of data to observe the interplay between cost, service and environmental resource efficiency in an integrated way: from a unit operations production processes perspective, a manufacturing footprint analysis and the final distribution of medicines to patients - rather than the functional ‘silos’ in which new technologies are often assessed”
The workstream also analysed ‘last-mile’ logistics, with global pharmacy Alliance Healthcare, and the life sciences division of the logistics specialist, DHL, as part of the ReMediES project. Adopting approaches used by the IfM researchers in FMCG and retail distribution logistics, the team modelled direct supply to patients to evaluate feasibility in terms of cost and service levels.
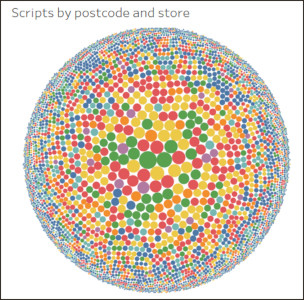
Visualisation of numbers of prescriptions being processed by pharmacists in selected postcodes over the course of a month
It has not all been hard work and no play – the team have, for example, used gamification to develop a mobile app helping industry experts and multiple supply chain stakeholders within the ReMediES programme to evaluate otherwise complex risk interdependencies across the extended supply chain. This work links well with a broader disruption risk assessment tool available from ReMediES partner Intersys, now marketed commercially.
What next?
The work of ReMediES will continue through the new £56 million Medicines Manufacturing Innovation Centre (MMIC), located in Glasgow, which is jointly funded by Innovate UK, Scottish Enterprise, GSK and AstraZeneca. MMIC is designed to help both start-ups and multinational pharmaceutical companies adopt novel processes and technologies and customise them to integrate with their own manufacturing processes. Just-in-time clinical pharmacy and continuous direct compression are MMIC’s first ‘Grand Challenges’, taking forward the work of two of ReMediES’s core projects.
Forging a model for collaboration
Whilst new technologies are offering major opportunities to patients, healthcare providers and manufacturers, they require new collaborative platforms to address the complex technical and regulatory challenges that are involved. It’s too big to do alone.
However, the concept of pre-competitive collaboration is relatively new in the medicines manufacturing arena, and many companies are concerned about becoming engaged from a cost and IP perspective. ReMediES addresses this concern in part by providing an exemplar collaboration model where innovations emerging from fundamental and applied research can be taken to prototype or commercialisation. The collaboration has enabled multi-stakeholder perspectives to be considered, drawing in expertise from technology providers, regulators, academia and industry. Individual firms have been able to leverage their resources, upwards of 50% on direct activity, and by over 300% at a programme level.
Dr Will Barton, Chairman of the Advisory Board of the EPSRC Centre for Innovative Manufacturing in Additive Manufacturing, was the independent advisor to the ReMediES Project. He comments:
“ReMediES was one of the early examples of the pharmaceutical industry working together in collaborative R&D, something it didn’t have a record of doing before. The project has shown the value of such collaboration, that you don’t have to share your crown jewels and that there are a lot of areas where companies can work together if they choose to, which can benefit the whole industry.
“ReMediES was a well-run project that will deliver some early wins for the SMEs and longer-term impacts across the board. It was really exciting seeing teams deliver great results. Things happened that nobody would have believed possible at the start.”
For further information please contact:
Dr Jagjit Singh Srai









